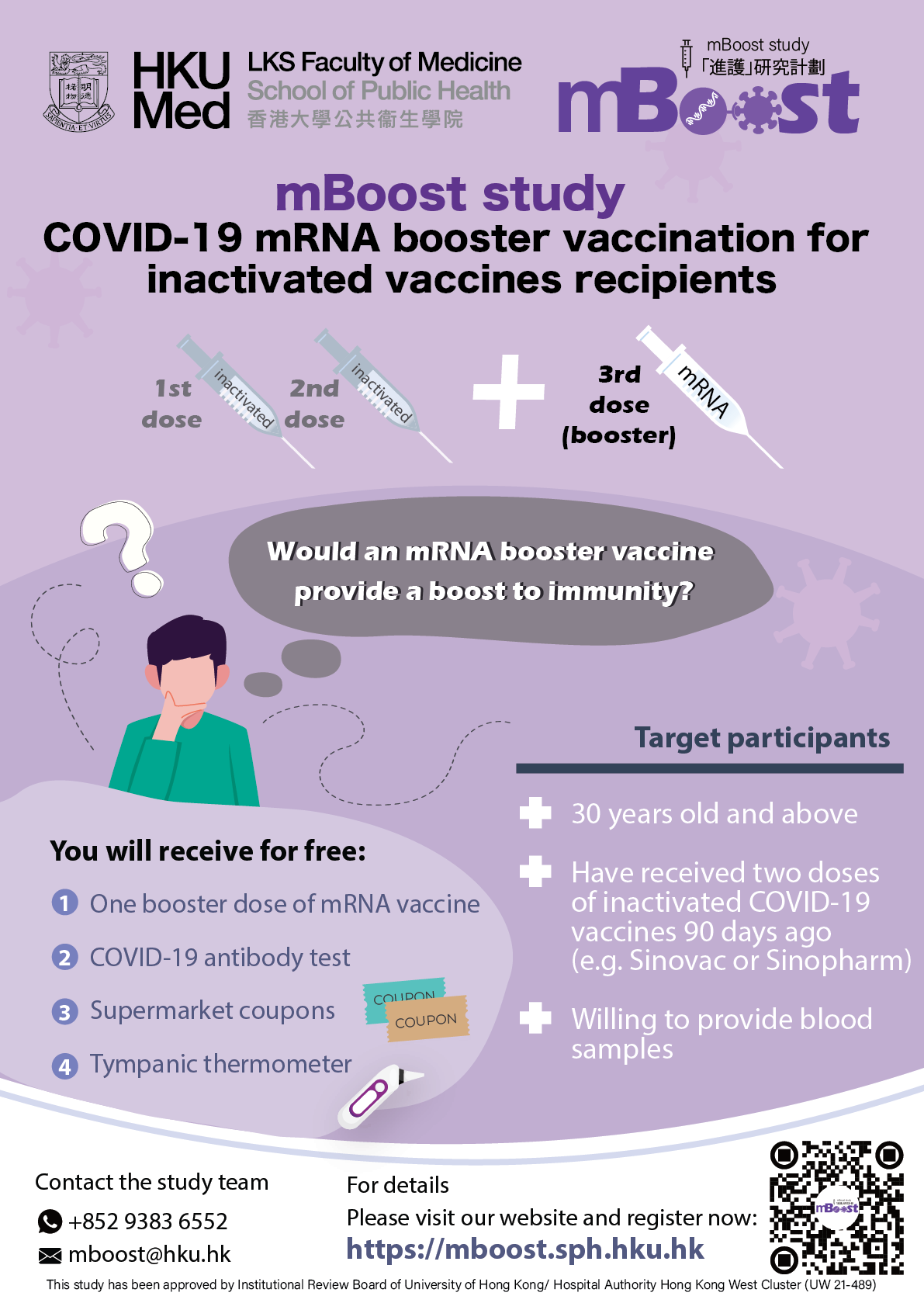
- 18 years old or above
- Have received two doses of BioNTech (Comirnaty) OR two doses of Sinovac (CoronaVac), with the most recent dose at least six months (180 days) prior to enrolment
- Willing to provide blood samples
- A history of confirmed COVID-19 infection
- Have received any type of COVID-19 vaccines other than Comirnaty or CoronaVac before
- You received the previous first and second dose of COVID-19 vaccine 43 days or more apart
- Have allergies to the active substance or other ingredients of mRNA or inactivated COVID-19 vaccines
- Have any medical condition related to the immune system as determined by a clinician
- Use of medication that impairs immune system in the last 6 months, except topical steroids or short-term oral steroids (course lasting 14 days or less)
- Have used any blood products within the past 90 days
- Currently pregnant, or planning for lactation or to become pregnant in the coming 3 months
- 18歲或以上人士
- 曾在至少6個月(180天)前接種了兩劑復必泰或科興疫苗
- 願意提供血液樣本
- 曾確診感染新冠病毒(COVID-19)
- 曾接種除了復必泰或科興疫苗以外的任何其他新冠疫苗
- 您已接種的第一劑及第二劑新冠疫苗相隔43日或以上
- 對mRNA新冠疫苗或滅活新冠疫苗之活性成分或其他成分過敏
- 有被醫生診斷與免疫系統相關的任何醫療狀況
- 在過去6個月內曾使用抑制免疫系統的藥物,外用類固醇或短期口服類固醇(療程持續14天或以下)除外
- 在過去90天內曾使用任何血液製品
- 正在懷孕,或計劃於未來3 個月內懷孕或哺乳
1.
If you are 18 years old or above, have received two doses of Sinovac (CoronaVac) or BioNTech (Comirnaty) 180 days ago, and interested to receive a 3rd booster dose of either an inactivated vaccine (CoronaVac) or a mRNA vaccine (Comirnaty) now
(Click “Join Now” )
Research personnel conduct initial screening on enrolment eligibility
Research personnel arrange a face-to-face interview with you for further assessment
1.
如您是18歲或以上人士、曾在至少180天前接種兩劑復必泰或科興疫苗、及有興趣現在接種一劑第三(加強)劑滅活疫苗(科興)或mRNA新冠疫苗(復必泰)
完成簡單網上問卷登記
(按此「立即參加」)
2.
研究人員就參與資格進行初步篩選
3.
研究人員聯絡您安排會面作進一步評估
During the interview, research personnel explain the details of the study to you and confirm your participation
Upon enrolment to the study, you will be randomised to receive either a (booster) dose of inactivated vaccine (CoronaVac) or mRNA vaccine (Comirnaty)
During the study period, regular follow-up activities include questionnaires and blood collection
4.
於會面期間,研究人員為您講解研究內容並確認參與
5.
納入研究時,您將會被隨機分配接種一劑(加強劑)滅活疫苗(科興)或mRNA新冠疫苗(復必泰)
6.
於研究期間,定期跟進包括問卷調查及抽血
No cost will be incurred for joining this study. To appreciate your support for our study, you will receive tympanic thermometer/ supermarket coupons during each regular follow-up for sample collection. In addition throughout the study, we may occasionally send you the results of your laboratory testing or findings of the study as a whole.
參與此研究費用全免。為答謝參加者對本研究的支持,參加者在每次定期跟進提供樣本後會獲贈耳探體溫計/超級市場禮券。另外於研究期間,研究團隊亦會不時向參加者發報其個人的檢測報告或本研究的整體研究結果。
如欲知道更多詳情,可於本網頁填寫及提交網上登記表格或直接聯絡本研究團隊。
- Click “Join Now” to complete a simple registration form online
- Due to popular demand, we aim to complete initial screening on enrolment eligibility and get back to you within two weeks after registration
- Call our study hotline during office hours
- 按此「立即參加」進行簡單網上登記
- 報名反應熱烈,我們目標兩星期內回覆參於資格初步篩選結果
- 於辦公時間內致電本研究熱線
Contact the research team
Cobovax study
School of Public Health, The University of Hong Kong
Phone/WhatsApp: +852 9446 3186
Email: cobovax@hku.hk
Office hours: Monday to Friday, 10am to 5pm (except public holidays)
聯絡研究團隊
Results
Pending
研究結果
待定
About Coronavirus Disease 2019 (COVID-19)
The recent emergence of the new coronavirus disease 2019 (COVID-19) in late 2019 and the subsequent global pandemic has since caused millions of deaths and significant economic impacts globally. COVID-19 is caused by the infection of SARS-CoV-2 coronavirus where disease severity varies widely, from asymptomatic infection, mild self-limiting disease with common respiratory symptoms, to severe disease requiring hospitalization that may lead to long-term symptoms or death.
About COVID-19 Vaccine
About vaccine booster shots
- in some more vulnerable groups such as immuocompromised individuals, compared to otherwise healthier individuals, the previous two doses of vaccination have not activated strong enough protective immune responses
- in any individuals, immune responses after vaccination may decrease over time naturally
- some COVID-19 vaccines may perform less well against new emerging virus strains
有關2019冠狀病毒病(COVID-19)
有關COVID-19疫苗
有關疫苗加強劑
- 在一些較脆弱的群體中如免疫力較弱人士,與其他更健康人士相比,在接種前兩劑新冠疫苗後可能並未能產生足夠強的免疫保護反應
- 在任何人士,接種疫苗後的免疫反應可能會隨著時間自然地下降
- 一些新冠疫苗對抵抗新出現的新冠病毒株的效用可能較差